Polio Guidance for Health Professionals
- Diagnosis
- Case definition
- Investigation
- Management
- Notifying and Reporting
- National polio preparedness and response plan
Diagnosis
The WHO defines a suspected case of polio as:
“…a child under 15 years of age presenting with acute flaccid paralysis, or as any person at any age with paralytic illness if poliomyelitis is suspected”
The case definition can be used to aid in determining possible, probable, or confirmed cases of poliomyelitis.
Immediate notification to the MOH is indicated for:
- All possible, probable, or confirmed cases
- Any adult with AFP who has any abnormalities on spinal MRI imaging
See Notifying and Reporting section below for details on criteria for reporting and how to report.
Investigation
Radiological Examination
All patients presenting with AFP should have MRI Brain and Full Spinal MRI. Classical acute flaccid myelitis (AFM) findings will normally be seen in spinal cord grey matter on T2-weighted imaging.
Sampling
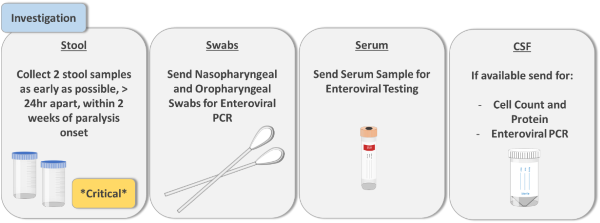
Four samples should be sent to exclude poliomyelitis:
1. Stool Sample:
- Two samples, 10 – 20 grams, 24hr apart within 14 days of onset of paralysis
- Label “Collected for enteroviral and polioviral testing for AFP case”
2. Serum:
- 1 x serum collection (Red/Green top), 1ml minimum
3. CSF:
- Perform only if CSF analysis being undertaken for other investigation. Poliovirus yield from CSF is low
- Normally a pleocytosis with increased WCC and mildly elevated protein (40 – 50 mg/dL) will be observed
4. Nasopharyngeal/Oropharyngeal Swab:
- Label “Collected for enteroviral PCR for AFP case”
Consultation with local laboratory is mandatory and all staff receiving samples must be informed in advance. All samples must be sent to local laboratory on ice, and transported to NVRL on dry ice to be kept < -20°C
All samples must be labelled as:
“INVESTIGATION OF AFP – ENTEROVIRAL TESTING, SEND TO NVRL”
Management
Suspected Cases of Poliomyelitis
Clinical Care
Management should be guided through consultation with specialist neurology and infectious diseases clinical services. Whilst management of AFM is largely supportive, advice can be found on the CDC website (https://www.cdc.gov/acute-flaccid-myelitis/hcp/clinical-management.html)
Isolation
All patients with suspected poliomyelitis must be isolated with separate toilet facilities from other patients. [All faeces should be collected and incinerated or inactivated to avoid release of infectious material into the environment]. Staff attending to patient must use droplet and contact precautions and must be vaccinated. Handwashing with soap and water is more effective at killing poliovirus than alcohol gel.
Confirmed Cases of Poliomyelitis
Clinical Care
Management of the index case should be guided by specialist neurology and infectious diseases clinical services.
Isolation
Patients with confirmed poliomyelitis must be isolated with separate toilet facilities. In addition, contaminated faeces must not be released into the environment and must instead be collected for incineration or inactivation. Staff attending to patient must use droplet and contact precautions and must be vaccinated. Handwashing with soap and water is more effective at killing poliovirus than alcohol gel. Stool samples should be collected weekly and sent for testing at the National Virus Reference Laboratory (NVRL). Isolation must continue until two stool samples taken 7 days apart are negative for poliovirus.
Management of Close Contacts
It is important to identify close contacts of the patient as early as possible. Ensure all close contacts have been vaccinated. Close contact details should be reported to the MOH. A close contact identification and management tool is provided below with details on the samples required from close contacts and the actions to be initiated. For full details on close contact management see The National Polio Plan.
There are 6 categories of close contact to be identified.
- Household and Sexual contacts
- Toilet contacts
- Food consumer contacts
- Facility first-aid workers or first responders
- Health care workers
- Sewerage workers
| Type of Contact | Description | Actions | Samples |
|---|---|---|---|
| Household and Sexual Contacts | Living with infected person and sharing toilet facilities. Greatest risk | Isolate at home with separate toilet facilities. Offer IPV vaccination. Booster if last vaccine > 10 years ago. Full vaccine course if uncertain status. |
Take stool samples 24 – 48 hours apart and more than 3 days after first contact. Two negative stool samples before leaving isolation |
| Toilet Contacts and Co-workers |
Sharing toilet facilities within 30 days before patient’s symptom onset | Offer IPV vaccination. Booster if last vaccine > 10 years ago. Full vaccine course if uncertain status. | |
| Food Consumer Contacts | Ate food prepared by infected person | Offer IPV vaccination. Booster if last vaccine > 10 years ago. Full vaccine course if uncertain status. | |
| Facility First-Aid Worker/First Responders | Provided assistance to infected person without PPE | Offer IPV vaccination. Booster if last vaccine > 10 years ago. Full vaccine course if uncertain status. |
|
| Healthcare Workers | Involved in patient care | Offer IPV vaccination. Booster if last vaccine > 10 years ago. Full vaccine course if uncertain status. | Take stool samples 24 – 48 hours apart and more than 3 days after first contact. |
| Sewerage Workers | Low risk, liaise with PH regarding wastewater surveillance | Offer IPV vaccination. Booster if last vaccine > 10 years ago. Full vaccine course if uncertain status. |
Notifying and Reporting
1. Notification
Immediate notification is mandated, and physicians are obliged to do so. Notification should be done before laboratory confirmation of poliovirus. Notify the MOH for all cases of AFP in patients < 15 years old. In adults, notify the MOH where polio is suspected, or in adults with AFP and any findings on spinal imaging. Completing “Reporting” or “Surveillance” forms should not delay notification. Contact details for appropriate MOH are detailed below.
List of Medical Officers of Health (MOH) by HSE Area
Notifying infectious diseases - Who to notify
Out of hours contact for DPH/MOH
Phone Ambulance Control and ask for Public Health On Call
2. Reporting and Surveillance
After initial notification using the standard notification form on the HPSC website, confirmed cases must be reported to the MOH, by completing the “Acute Flaccid Paralysis Questionnaire” available on the HPSC website. This is required as part of WHO global polio surveillance.
Last updated: 3 March 2023


